Articles from Cybin Inc.

Cybin Inc. (NYSE American:CYBN) (Cboe Canada CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to advancing mental healthcare by developing new and innovative next-generation treatment options, today announced that it has launched an at-the-market equity program (the “ATM Program”) to allow Cybin to issue and sell up to US$100,000,000 of common shares in the capital of the Company (the “Shares”) from treasury to the public, from time to time, through Cantor Fitzgerald and Co. and Cantor Fitzgerald Canada Corporation (together the “Agents”).
By Cybin Inc. · Via Business Wire · December 30, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN), a clinical-stage breakthrough pharmaceutical company committed to revolutionizing mental healthcare by developing innovative next-generation treatment options, today announced that it will voluntarily transfer its U.S. stock exchange listing to the Nasdaq Global Market (“Nasdaq”) from the NYSE American LLC (“NYSE American”). The Company expects that its common shares will cease trading on the NYSE American at market close on January 2, 2026 and commence trading on Nasdaq at market open on January 5, 2026.
By Cybin Inc. · Via Business Wire · December 19, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today reported unaudited financial results for its second quarter ended September 30, 2025, and recent business highlights.
By Cybin Inc. · Via Business Wire · November 13, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (”Cybin” or the “Company”), a breakthrough Phase 3 clinical stage neuropsychiatry company committed to revolutionizing mental healthcare through proprietary drug discovery platforms and innovative delivery systems, today announced that George Tziras, Cybin’s Chief Business Officer, will present at the Jefferies Global Healthcare Conference, taking place November 17-20, 2025, in London, UK.
By Cybin Inc. · Via Business Wire · November 12, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (”Cybin” or the “Company”), a breakthrough Phase 3 clinical stage neuropsychiatry company committed to revolutionizing mental healthcare through proprietary drug discovery platforms and innovative delivery systems, today announced that it will host a conference call and webcast at 8:00 a.m. ET on Thursday, November 13, 2025, to provide a business update and report financial results for its second quarter ended September 30, 2025.
By Cybin Inc. · Via Business Wire · November 6, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (”Cybin” or the “Company”), a breakthrough Phase 3 clinical stage neuropsychiatry company committed to revolutionizing mental healthcare through proprietary drug discovery platforms and innovative delivery systems, today announced that George Tziras, Cybin’s Chief Business Officer, will participate in a fireside chat at the Guggenheim 2nd Annual Healthcare Innovation Conference, taking place November 10-12, 2025, in Boston, MA.
By Cybin Inc. · Via Business Wire · November 5, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (”Cybin” or the “Company”), a breakthrough Phase 3 clinical stage neuropsychiatry company committed to revolutionizing mental healthcare through proprietary drug discovery platforms and innovative delivery systems, today announced that Amir Inamdar, Cybin’s Chief Medical Officer, will participate in a panel at the 2025 Milken Institute Future of Health Summit, taking place November 4-6, 2025, in Washington, D.C. The panel details are as follows:
By Cybin Inc. · Via Business Wire · November 3, 2025

Cybin Inc. (Cboe CA: CYBN) (NYSE American: CYBN) (“Cybin” or the “Company”), a breakthrough Phase 3 clinical stage neuropsychiatry company committed to revolutionizing mental healthcare through proprietary drug discovery platforms and innovative delivery systems, is pleased to announce that it has closed its previously announced registered direct offering of 22,277,750 common shares in the capital of the Company (a “Common Share”) and, in lieu of Common Shares to certain investors, 4,605,500 pre-funded Common Share purchase warrants (the “Pre-Funded Warrant”) at a price of US$6.51 per Common Share or Pre-Funded Warrant for aggregate gross proceeds of US$175,009,911.45 (the “Offering”).
By Cybin Inc. · Via Business Wire · October 31, 2025

Cybin Inc. (Cboe CA: CYBN) (NYSE American: CYBN) (“Cybin” or the “Company”), a breakthrough Phase 3 clinical stage neuropsychiatry company committed to revolutionizing mental healthcare through proprietary drug discovery platforms and innovative delivery systems, is pleased to announce a registered direct offering of 22,277,750 common shares in the capital of the Company (a “Common Share”) and, in lieu of Common Shares to certain investors, pre-funded Common Share purchase warrants (the “Pre-Funded Warrant”) at a price of US$6.51 per Common Share or Pre-Funded Warrant for aggregate gross proceeds of US$175,009,911.45 (the “Offering”).
By Cybin Inc. · Via Business Wire · October 28, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (”Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare through proprietary drug discovery platforms and innovative delivery systems, today highlights significant clinical and regulatory milestones and upcoming value-driving catalysts as the Company advances multiple programs towards potential commercialization.
By Cybin Inc. · Via Business Wire · September 23, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that Amir Inamdar, Cybin’s Chief Medical Officer, and George Tziras, Cybin’s Chief Business Officer, will participate in a fireside chat at TD Cowen's 5th Annual Novel Mechanisms in Neuropsychiatry & Epilepsy Summit on Wednesday, September 17, 2025, at 9:20 a.m. ET.
By Cybin Inc. · Via Business Wire · September 11, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to advancing mental healthcare by developing new and innovative next-generation treatment options, today announced completion of enrollment in its Phase 2 study evaluating CYB004, a proprietary deuterated dimethyltryptamine (“DMT”) program, for the treatment of Generalized Anxiety Disorder (“GAD”) and reaffirms top line data guidance expected in the first quarter of 2026.
By Cybin Inc. · Via Business Wire · September 8, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that George Tziras, Cybin’s Chief Business Officer, will participate in a fireside chat at the H.C. Wainwright 27th Annual Global Investment Conference on Monday, September 8, 2025, at 11:00 a.m. ET.
By Cybin Inc. · Via Business Wire · September 4, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that Amir Inamdar, Cybin’s Chief Medical Officer, will participate in a fireside chat at the Cantor Global Healthcare Conference, taking place September 3-5, 2025.
By Cybin Inc. · Via Business Wire · September 3, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe Canada:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, announced that, effective September 2, 2025, Doug Drysdale will step down as the Company’s Chief Executive Officer. The Company’s Co-Founder and President, Eric So, has been appointed as Interim Chief Executive Officer by the Board of Directors.
By Cybin Inc. · Via Business Wire · September 2, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to advancing mental healthcare by developing new and innovative next-generation treatment options, today announced that it has received approval in Australia to conduct the EMBRACE® study. EMBRACE is the second pivotal study in PARADIGM™, the Company’s Phase 3 multinational program evaluating CYB003, a proprietary deuterated psilocin analog. The Company has received approval through the Clinical Trial Notification scheme, obtained clearance from multiple Ethics Committees of the Australian Therapeutics Goods Administration, and the study site Research Governance Offices, thus allowing the commencement of the EMBRACE study.
By Cybin Inc. · Via Business Wire · August 26, 2025

Cybin Inc. (NYSE American: CYBN) (Cboe CA: CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to advancing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce the voting results for each of the matters presented at the Company’s annual meeting of shareholders held on August 18, 2025 (the “Meeting”). There were 90 shareholders represented in person or by proxy at the Meeting holding 11,264,212 common shares, representing 48.92% of Cybin’s total issued and outstanding common shares as at the record date for the Meeting. The voting results for each matter presented at the Meeting are set out below:
By Cybin Inc. · Via Business Wire · August 18, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe Canada CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today reported unaudited financial results for its first quarter ended June 30, 2025, and is pleased to provide an update on key business milestones.
By Cybin Inc. · Via Business Wire · August 13, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to advancing mental healthcare by developing new and innovative next-generation treatment options, today announced that its Clinical Trial Application (“CTA”) has been approved by the Irish Medicines Board, acting as the reference Member state, to initiate the EMBRACE™ study in Ireland, Poland, and Greece. EMBRACE is the second pivotal study in PARADIGM®, the Company’s Phase 3 multinational program evaluating CYB003, a proprietary deuterated psilocin analog. The Company also recently announced approval from the Medical and Healthcare products Regulatory Agency (“MHRA”) to commence EMBRACE in the United Kingdom.
By Cybin Inc. · Via Business Wire · August 7, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in a fireside chat at the Canaccord Genuity 45th Annual Growth Conference, on August 12, 2025 in Boston, MA.
By Cybin Inc. · Via Business Wire · August 6, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to advancing mental healthcare by developing new and innovative next-generation treatment options, today announced that it has received approval from the UK Medical and Healthcare Products Regulatory Agency (“MHRA”) to commence EMBRACE, the second pivotal study in PARADIGM, the Company’s Phase 3 multinational program evaluating CYB003, a proprietary deuterated psilocin analog. The Company previously received Breakthrough Therapy Designation from the U.S. Food and Drug Administration (“FDA”) for CYB003 for the adjunctive treatment of Major Depressive Disorder (“MDD”).
By Cybin Inc. · Via Business Wire · July 17, 2025

Cybin Inc. (NYSE American: CYBN) (Cboe Canada: CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce the Company has entered into a securities purchase agreement (the “Securities Purchase Agreement”) with High Trail Special Situations LLC (“High Trail”), pursuant to which the Company agreed to sell and issue to High Trail up to US$500,000,000 in aggregate principal amount of unsecured convertible debentures (the “Convertible Debentures”). The sale and issue of US$50,000,000 principal amount of Convertible Debentures was completed on June 30, 2025 (the “Private Placement”). The sale and issue of US$450,000,000 of the principal amount of Convertible Debentures will be determined at a future date, upon mutual agreement of the parties.
By Cybin Inc. · Via Business Wire · July 1, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe Canada CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today reported audited financial results for its fiscal year ended March 31, 2025, and recent business highlights.
By Cybin Inc. · Via Business Wire · June 30, 2025

Cybin Inc. (NYSE American: CYBN) (Cboe Canada: CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce the Company has entered into a securities purchase agreement (the “Securities Purchase Agreement”) with High Trail Special Situations LLC (“High Trail”), pursuant to which the Company agreed to sell and issue to High Trail up to US$500,000,000 in aggregate principal amount of unsecured convertible debentures (the “Convertible Debentures”). The sale and issue of US$50,000,000 principal amount of Convertible Debentures was completed on June 30, 2025 (the “Private Placement”). The sale and issue of US$450,000,000 of the principal amount of Convertible Debentures will be determined at a future date, upon mutual agreement of the parties.
By Cybin Inc. · Via Business Wire · June 30, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe Canada CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today provided a corporate update.
By Cybin Inc. · Via Business Wire · June 13, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in a virtual fireside chat at the H.C. Wainwright 6th Annual Neuro Perspectives Conference, taking place June 16-17, 2025.
By Cybin Inc. · Via Business Wire · June 12, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will be speaking at the Psychedelic Science 2025 Conference taking place June 16-20, 2025 in Denver, Colorado. The details are as follows:
By Cybin Inc. · Via Business Wire · June 11, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that the United States Patent and Trademark Office has granted U.S. patent 12,318,477 in support of its CYB004 deuterated DMT program in development for the treatment of generalized anxiety disorder (“GAD”).
By Cybin Inc. · Via Business Wire · June 3, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in a Water Tower Research Fireside Chat taking place on Thursday, May 29, 2025, at 11:00 a.m. ET.
By Cybin Inc. · Via Business Wire · May 22, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, applauds recent comments made by Dr. Martin Makary, Commissioner of the U.S. FDA relating to the importance of accelerating and prioritizing research on the clinical benefits of psychedelic therapeutics.
By Cybin Inc. · Via Business Wire · May 20, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that it has engaged Thermo Fisher Scientific (“Thermo Fisher”), the world leader in serving science, to support the Phase 3 clinical supply and potential commercial manufacturing of CYB003, the Company’s proprietary deuterated psilocin program in Phase 3 development for the adjunctive treatment of Major Depressive Disorder (“MDD”).
By Cybin Inc. · Via Business Wire · May 15, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in a fireside chat at the virtual Alliance Global Partners Healthcare Company Showcase, taking place May 21, 2025.
By Cybin Inc. · Via Business Wire · May 14, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry platform company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that the United States Patent and Trademark Office has granted U.S. patent 12,291,499 in support of its CYB003 program in MDD.
By Cybin Inc. · Via Business Wire · May 8, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that Doug Drysdale, Cybin’s Chief Executive Officer, will be speaking at the 28th Annual Milken Institute Global Conference on a panel entitled “The Global Landscape and Opportunities for Medical Breakthroughs.” The Conference will take place May 4-7, 2025, at the Beverly Hilton in Los Angeles, and the panel will be held on Monday, May 5, 2025, at 2:30 p.m. PDT.
By Cybin Inc. · Via Business Wire · May 1, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry platform company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced additional strategic partnership agreements (“SPAs”), bringing the total to 18 clinical sites engaged to advance Cybin’s multinational Phase 3 program evaluating CYB003 for the adjunctive treatment of MDD. The APPROACH study is expected to include approximately 45 clinical sites.
By Cybin Inc. · Via Business Wire · April 23, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced a strategic partnership with Osmind, a leading service provider advancing psychiatry through technology, services, and real-world evidence to bring innovative mental health treatments to patients in need.
By Cybin Inc. · Via Business Wire · April 21, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe Canada CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today reported unaudited financial results for its third quarter ended December 31, 2024, and recent business highlights.
By Cybin Inc. · Via Business Wire · February 10, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry platform company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced the launch of its first strategic partnership agreement (“SPA”) with Segal Trials in furtherance of Cybin’s multinational pivotal Phase 3 program evaluating CYB003 for the adjunctive treatment of Major Depressive Disorder (“MDD”).
By Cybin Inc. · Via Business Wire · January 15, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to provide a year-end summary of its key accomplishments in 2024 and upcoming milestones for 2025.
By Cybin Inc. · Via Business Wire · January 13, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in a fireside chat at the Lytham Partners 2025 Investor Healthcare Summit taking place virtually on January 13, 2025.
By Cybin Inc. · Via Business Wire · January 9, 2025

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced the presentation of two posters at the American College of Neuropsychopharmacology (“ACNP”) Annual Meeting taking place December 8-11, 2024, in Phoenix, Arizona.
By Cybin Inc. · Via Business Wire · December 10, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, and Amir Inamdar, MBBS, DNB (Psych), FFPM, Cybin’s Chief Medical Officer, will participate in the Water Tower Research Fireside Chat Series taking place on Wednesday, December 11, 2024, at 11:00 a.m. ET.
By Cybin Inc. · Via Business Wire · December 4, 2024

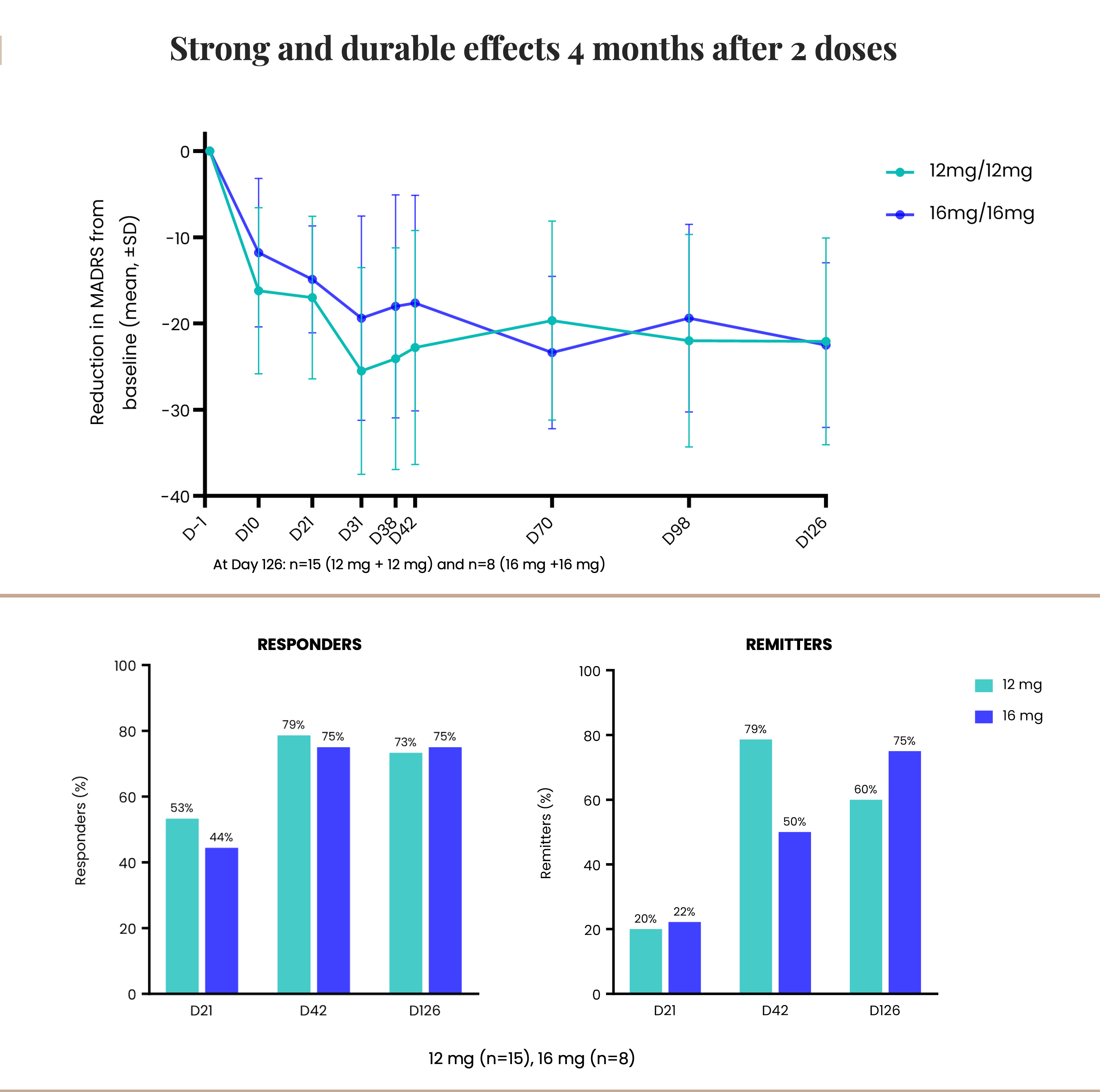
Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced unprecedented 12-month efficacy data from its Phase 2 study of CYB003, a proprietary deuterated psilocin program in development for the potential adjunctive treatment of major depressive disorder (“MDD”). As previously announced, CYB003 received Breakthrough Therapy Designation by the U.S Food and Drug Administration (the “FDA”) for this indication, which provides an expedited review pathway.
By Cybin Inc. · Via Business Wire · November 18, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry platform company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that it will host a conference call and webcast on Monday, November 18, 2024 at 8:00 a.m. ET.
By Cybin Inc. · Via Business Wire · November 14, 2024
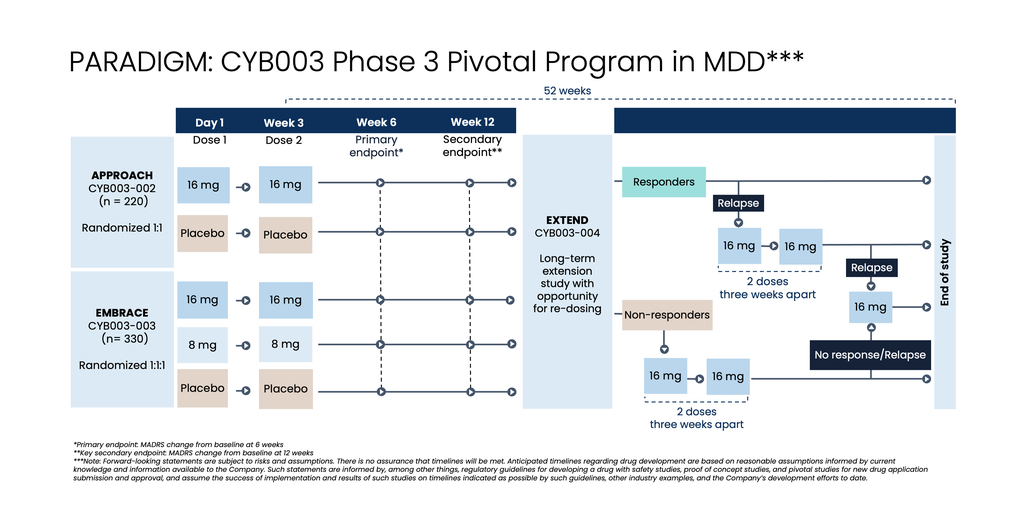
Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced the initiation of PARADIGMTM, its Phase 3 pivotal program evaluating the efficacy and safety of CYB003 for the adjunctive treatment of Major Depressive Disorder (“MDD”). The program name, PARADIGM, represents the Company's belief that CYB003 could have the potential for a paradigm shift in the treatment of depression. The Company also today reported unaudited financial results for its second quarter ended September 30, 2024.
By Cybin Inc. · Via Business Wire · November 13, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry platform company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that Doug Drysdale, Cybin’s Chief Executive Officer, will be speaking at the 2024 Milken Institute Future of Health Summit on a panel entitled “The Next Frontier in Mental Health Research.”
By Cybin Inc. · Via Business Wire · October 31, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry platform company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that the United States Patent and Trademark Office (“USPTO”) has granted U.S. patent 12,122,741 (‘741) with claims to the composition of matter of lead preclinical candidates in the Company's CYB005 phenethylamines program.
By Cybin Inc. · Via Business Wire · October 24, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in the Water Tower Research Fireside Chat Series taking place on Tuesday, October 8, 2024, at 3:00 p.m. ET.
By Cybin Inc. · Via Business Wire · October 3, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced the appointment of senior clinical team leaders, Dr. Mirza Rahman as Senior Vice President, Patient Safety & Pharmacovigilance and Dr. Marcelo Gutierrez, Vice President, Clinical Pharmacology, as well as the expansion of its clinical operations team.
By Cybin Inc. · Via Business Wire · October 1, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Amir Inamdar, Cybin’s Chief Medical Officer, will participate in a panel discussion at the TD Cowen 4th Annual Novel Mechanisms in Neuropsychiatry Summit taking place virtually on September 26, 2024.
By Cybin Inc. · Via Business Wire · September 24, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today reported recent clinical accomplishments and key upcoming clinical milestones.
By Cybin Inc. · Via Business Wire · September 19, 2024

Cybin Inc. (NYSE American: CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that it has filed articles of amendment to consolidate the Company’s issued and outstanding common shares (the “Common Shares”) on the basis of one new Common Share for every 38 existing Common Shares (the “Consolidation”). The Consolidation is effective immediately and trading of the Common Shares reflecting the Consolidation will commence at the opening of trading today.
By Cybin Inc. · Via Business Wire · September 19, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in a fireside chat and panel at the H.C. Wainwright 26th Annual Global Investment Conference, taking place September 9-11, 2024 in New York, NY.
By Cybin Inc. · Via Business Wire · September 4, 2024

Cybin Inc. (NYSE American: CYBN) (Cboe CA:CYBN) (Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce the voting results for each of the matters presented at the Company’s annual and special meeting of shareholders held on August 27, 2024 (the “Meeting”). There were 87 shareholders represented in person or by proxy at the Meeting holding 453,195,063 common shares, representing 59.66% of Cybin’s total issued and outstanding common shares as at the record date for the Meeting. The voting results for each matter presented at the Meeting are set out below:
By Cybin Inc. · Via Business Wire · August 27, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced that it held a Type B Initial Breakthrough Therapy Meeting with the U.S. Food and Drug Administration (“FDA”) late last week in Washington, D.C. The Company plans to initiate its Phase 3 pivotal trial of CYB003 for the adjunctive treatment of MDD in late summer of 2024.
By Cybin Inc. · Via Business Wire · August 13, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today reported unaudited financial results for its first quarter ended June 30, 2024, and recent business highlights.
By Cybin Inc. · Via Business Wire · August 8, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in a fireside chat at the Canaccord Genuity 44th Annual Growth Conference, taking place August 13-14, 2024 in Boston, MA.
By Cybin Inc. · Via Business Wire · August 7, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options for mental health disorders, today reported audited financial results for its fiscal year ended March 31, 2024, and recent business highlights.
By Cybin Inc. · Via Business Wire · June 26, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in a fireside chat at the H.C. Wainwright 5th Annual Neuro Perspectives Virtual Conference, taking place June 27, 2024.
By Cybin Inc. · Via Business Wire · June 19, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, is pleased to announce that Dr. Atul R. Mahableshwarkar M.D., DLFAPA, has joined the Company as Senior Vice President, Clinical Development. Dr. Mahableshwarkar will lead the development of the CYB003 program, Cybin’s proprietary deuterated psilocybin analog which has received U.S. Food and Drug Administration (“FDA”) Breakthrough Therapy Designation for the adjunctive treatment of major depressive disorder.
By Cybin Inc. · Via Business Wire · June 11, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced that Amir Inamdar, MBBS, DNB (Psych), MFPM, Cybin’s Chief Medical Officer, and Ellen James, Ph.D., Cybin’s Director, Clinical Development, will participate at the Interdisciplinary Conference on Psychedelic Research (“ICPR”) taking place June 6-8, 2024, in Haarlem, Amsterdam.
By Cybin Inc. · Via Business Wire · May 29, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will participate in a fireside chat at the virtual Alliance Global Partners Healthcare Company Showcase, taking place May 21, 2024.
By Cybin Inc. · Via Business Wire · May 14, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, provides a corporate update highlighting recent clinical accomplishments and key upcoming catalysts across its development pipeline.
By Cybin Inc. · Via Business Wire · May 6, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced that Doug Drysdale, Cybin’s Chief Executive Officer, will be speaking at the 27th Annual Milken Institute Global Conference on a panel entitled “Collaborating for Improved Mental Health.” The Conference will take place May 5-8, 2024, at the Beverly Hilton in Los Angeles, and the panel will be held on Wednesday, May 8, 2024, at 10:00 a.m. PDT.
By Cybin Inc. · Via Business Wire · April 25, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced that its research manuscript, entitled “Synthesis and Structure-Activity Relationships of 2,5-dimethoxy-4-substituted phenethylamines and the discovery of CYB210010: A potent, orally bioavailable and long-acting serotonin 5-HT2 receptor agonist,” has been published in the Journal of Medicinal Chemistry, a prestigious bi-weekly peer-reviewed publication.
By Cybin Inc. · Via Business Wire · April 18, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced that the United States Patent and Trademark Office has granted U.S. patent 11,958,807 in support of its CYB003 program in Major Depressive Disorder (“MDD”).
By Cybin Inc. · Via Business Wire · April 16, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will present at the 2024 Bloom Burton & Co. Healthcare Investor Conference, taking place April 16-17, 2024 in Toronto, Ontario.
By Cybin Inc. · Via Business Wire · April 10, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced participation in the upcoming Public Ventures Discovery Day on March 19, 2024 in Dallas, TX.
By Cybin Inc. · Via Business Wire · March 18, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced the initiation of a Phase 2 proof-of-concept study of CYB004, its proprietary DMT molecule in development for the treatment of GAD. In January 2024, the U.S. Food and Drug Administration (“FDA”) cleared Cybin’s Investigational New Drug application for CYB004.
By Cybin Inc. · Via Business Wire · March 15, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced a positive End-of-Phase 2 meeting with the FDA for CYB003, its deuterated psilocybin analog for the adjunctive treatment of Major Depressive Disorder (“MDD”).
By Cybin Inc. · Via Business Wire · March 14, 2024
Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced that the FDA has granted BTD to CYB003, its proprietary deuterated psilocybin analog in development for the adjunctive treatment of MDD. If approved by the FDA, CYB003 would be the first known adjunctive psychedelic-based therapeutic for the treatment of MDD.
By Cybin Inc. · Via Business Wire · March 13, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced that it will host a conference call and webcast at 8:30 a.m. ET on Wednesday, March 13, 2024.
By Cybin Inc. · Via Business Wire · March 12, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic treatment options, is pleased to announce that Doug Drysdale, Cybin’s Chief Executive Officer, will present at the TD Cowen 44th Annual Health Care Conference, taking place March 4-6, 2024 in Boston, MA.
By Cybin Inc. · Via Business Wire · February 27, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic treatment options, today reported unaudited financial results for its third quarter ended December 31, 2023, and recent business highlights.
By Cybin Inc. · Via Business Wire · February 14, 2024

Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage biopharmaceutical company committed to revolutionizing mental healthcare by developing new and innovative next-generation psychedelic-based treatment options, today announced that the Japan Patent (“JP”) Office has granted JP patents 2023-500532 and 2023-533436.
By Cybin Inc. · Via Business Wire · February 7, 2024
