Articles from Rapid Medical

Rapid Medical™, a leading developer of active endovascular devices, announces the completion of patient enrollment in the DISTALS Trial, a multicenter randomized study assessing the TIGERTRIEVER 13™ device in patients with distal medium vessel occlusions (DMVO) in acute ischemic stroke.
By Rapid Medical · Via Business Wire · December 9, 2025

Rapid Medical™, a leading developer of active endovascular devices, today announced the appointment of Nir Nimrodi to its Board of Directors. Nimrodi brings more than three decades of global leadership in the biotechnology and life sciences industries, with extensive experience in strategic growth, commercialization, and M&A for high-impact healthcare companies.
By Rapid Medical · Via Business Wire · September 25, 2025

Rapid Medical™, a leading developer of active endovascular devices, announces that its DRIVEWIRE™ 24 steerable guidewire has been used in more than 1,000 neurovascular procedures in North America during a limited commercial launch beginning earlier this year. The company also announced receipt of CE Mark under the European Medical Device Regulation (MDR), as presented at the 2025 ESMINT Annual Meeting.
By Rapid Medical · Via Business Wire · September 3, 2025

Rapid Medical™, a leading developer of active endovascular devices, announces the first patient enrolled in its COGNITIVE Study–the first to examine a link between mechanical thrombectomy and cognitive improvement. TIGERTRIEVER, the only active stent retriever, may uniquely preserve cognitive function with a tailored approach to removing thrombus while limiting impact to the natural vessel. National Principal Investigator Dr. Fawaz Al-Mufti, MD of Westchester Medical Center in Westchester, NY, performed the procedure.
By Rapid Medical · Via Business Wire · January 29, 2025

Rapid Medical™, a leading developer of advanced endovascular devices, announces completion of the interim safety analysis of the DISTALS Study at the 2024 Congress of the European Society of Minimally Invasive Neurological Therapy (ESMINT) Congress.
By Rapid Medical · Via Business Wire · September 4, 2024

Rapid Medical™, a leading developer of advanced endovascular devices, announces the first procedures in the USA with the breakthrough deflectable access platform DRIVEWIRE 24 at the 2024 Society of NeuroInterventional Surgery’s (SNIS) 21st Annual Meeting. With active technology, DRIVEWIRE articulates a wide range of catheters for direct access to endovascular locations.
By Rapid Medical · Via Business Wire · July 22, 2024
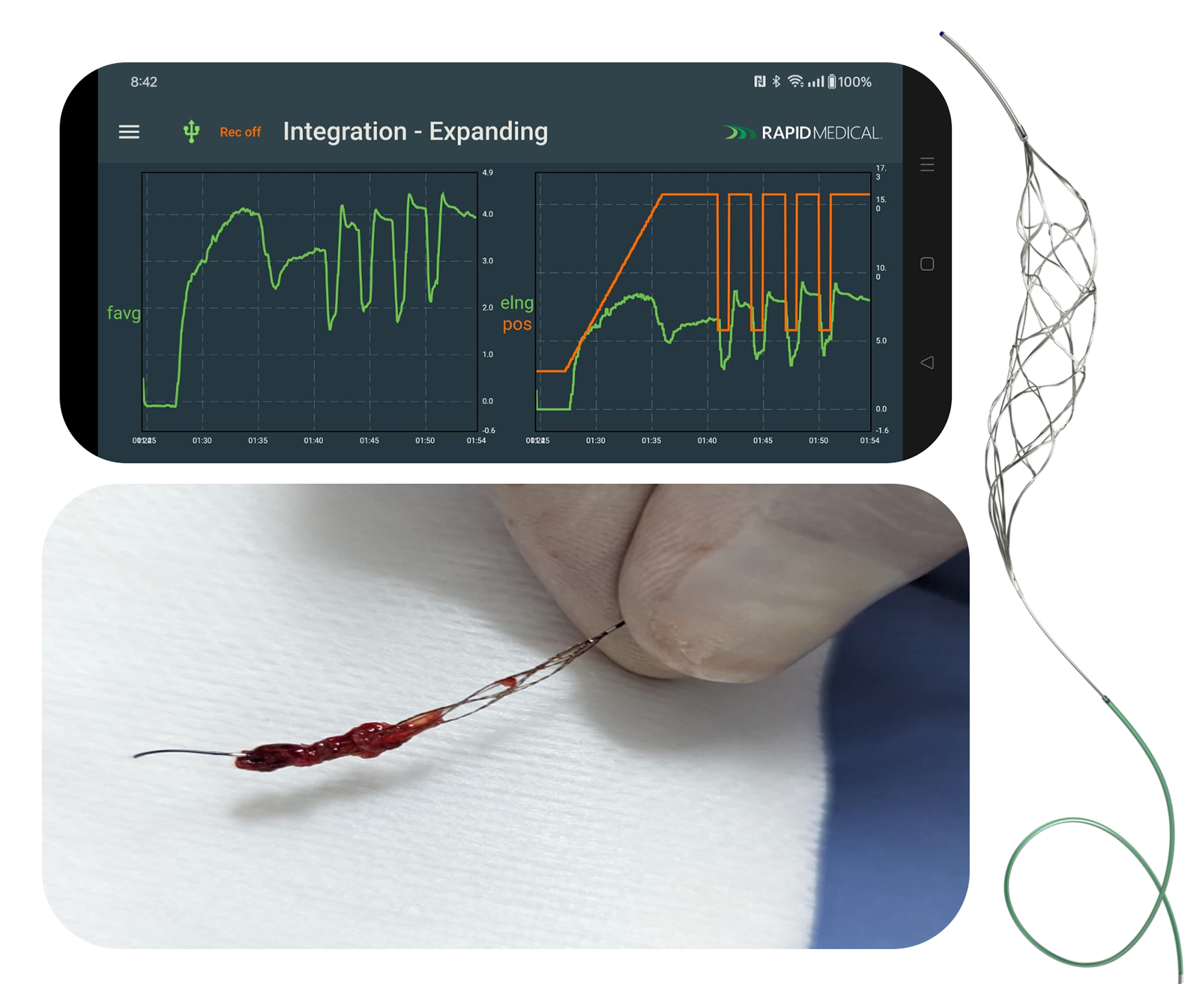
Rapid Medical™, a leading developer of advanced neurovascular devices, announces the first successful robotic thrombectomy in Medellín, Colombia. Two patients were treated with the Robotic TIGERTRIEVER™, the first endovascular thrombectomy device that adapts autonomously to the patient’s anatomy.
By Rapid Medical · Via Business Wire · May 14, 2024

Rapid Medical™, a leading developer of advanced neurovascular devices, announced Japanese approval for its TIGERTRIEVER revascularization device. With Pharmaceuticals and Medical Devices Agency (PMDA) approval, TIGERTRIEVER serves as the first device to offer individualized solutions for mechanical thrombectomy.
By Rapid Medical · Via Business Wire · December 13, 2023

Rapid Medical™, a leading developer of advanced neurovascular devices, announces new data demonstrating excellent first-pass treatment success with the TIGERTRIEVER™ device in complex ischemic stroke patients with underlying intracranial atherosclerotic disease (ICAD). The complication rates were negligible, as reported at the 2023 Society of Vascular and Interventional Surgery’s (SVIN) Annual Meeting.
By Rapid Medical · Via Business Wire · November 16, 2023

Rapid Medical™, a leading developer of advanced neurovascular devices, announced Chinese approval for its TIGERTRIEVER revascularization device. With this milestone of National Medical Product Administration (NMPA) approval, TIGERTRIEVER becomes the first device to offer patient-specific solutions for removing blood clots from the brain to advance the treatment of ischemic stroke.
By Rapid Medical · Via Business Wire · August 31, 2023

Rapid Medical™, a leading developer of advanced neurovascular devices, today announced new clinical data showing significant advantages of its novel COMANECI™ embolization assist device over established techniques to treat ruptured wide-neck intracranial aneurysms. A recent meta-analysis published in World Neurosurgery found that COMANECI is associated with lower hemorrhagic and thromboembolic complication rates, higher complete occlusion rates, and similar residual retreatment rates than stent-assisted and balloon-assisted coiling techniques.
By Rapid Medical · Via Business Wire · July 19, 2023

Rapid Medical, a leading developer of advanced neurovascular devices, announces FDA 510(k) clearance for TIGERTRIEVER™13 for large vessel occlusions at the 2022 Society of NeuroInterventional Surgery’s (SNIS) 19th Annual Meeting in Toronto. TIGERTRIEVER 13 is the smallest revascularization device in the world to date and is designed to remove thrombus from delicate brain blood vessels during an ischemic stroke. It is the only device that adjusts to the vasculature and clot, a more atraumatic approach than existing devices.
By Rapid Medical · Via Business Wire · July 26, 2022

Rapid Medical, a leading developer of advanced neurovascular devices, announces the expansion of its portfolio in the United States and the first Numen™ coil embolization procedure. Rapid Medical now has an exclusive distribution agreement with MicroPort NeuroTech, a subsidiary of MicroPort Scientific Corporation. MircoPort’s global presence offers nearly 300 medical solutions to patients in more than 80 countries and generates over $780 M in annual revenue.
By Rapid Medical · Via Business Wire · June 28, 2022

Rapid Medical, a leading developer of advanced neurovascular devices, announced today the enrollment of the first patient in the DISTALS Study. The trial is the first-ever FDA investigational device exemption (IDE) trial to examine the safety and effectiveness of mechanical thrombectomy in distal stroke.
By Rapid Medical · Via Business Wire · June 2, 2022

Rapid Medical, a leading developer of advanced neurovascular devices, today announced FDA breakthrough designation for its Comaneci™ embolization assist device to facilitate the treatment of cerebral vasospasm following hemorrhagic stroke. Vasospasm is a major complication and cause of morbidity. The FDA’s Breakthrough Device Program is designed to give patients more timely access to novel technologies, like Comaneci, that provide more effective treatments for life-threatening or irreversibly debilitating human diseases and conditions.
By Rapid Medical · Via Business Wire · February 15, 2022
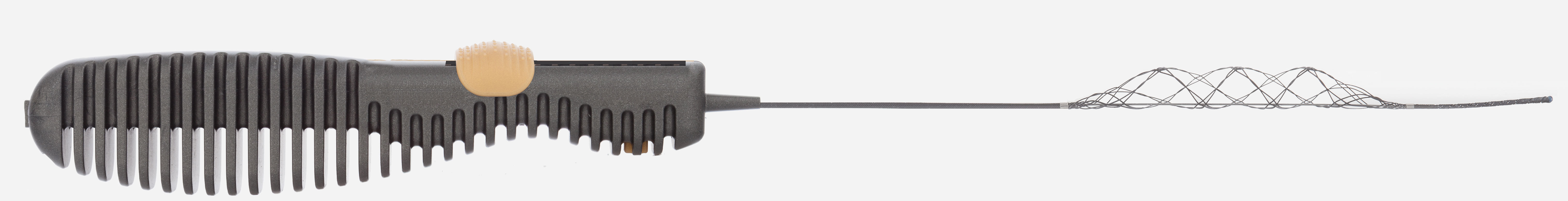
Rapid Medical, a leading developer of advanced neurovascular devices, today announced FDA investigational device exemption (IDE) approval for the first-ever trial to expand interventional stroke treatment to distal regions of the brain. The DISTALS Study is a pivotal, international, multi-center, randomized controlled trial (RCT) to evaluate the safety and effectiveness of distal thrombectomy. This new frontier for ischemic stroke has been created by Rapid Medical’s development of TIGERTRIEVER™ 13, the smallest thrombectomy device available.
By Rapid Medical · Via Business Wire · November 3, 2021

Rapid Medical, a company focused on the development of responsive, adjustable neurovascular devices, announced today that Daniel Levangie has been appointed to its Board of Directors.
By Rapid Medical · Via Business Wire · July 13, 2021

Rapid Medical, a company focused on the development of responsive, adjustable neurovascular devices, announced today that it has completed an oversubscribed Series D financing of $50M. The proceeds will be used to support the fast commercial growth of the company’s minimally invasive stroke products worldwide. The round was led by MicroPort with participation from CITIC private equity fund (CPE), Deep Insight and existing investors.
By Rapid Medical · Via Business Wire · May 25, 2021

The first patients in the United States have been treated with Rapid Medical’s FDA cleared TIGERTRIEVER™ revascularization device by Dr. Rishi Gutpa, Director of Neurocritcal Care at Wellstar Health System in Georgia. TIGERTRIEVER is the first adjustable stent retriever, enabling neuro interventionalists to better remove blood clots and restore blood flow to the brain following an ischemic event—a condition that devastates 800,000 Americans annually.
By Rapid Medical · Via Business Wire · May 5, 2021
